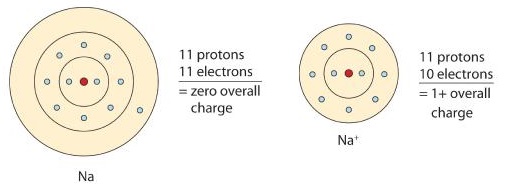
If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. It is now referred to as a sodium ion. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. Solvated electrons are involved in the reaction of sodium metal with water. Two solvated electrons combine to form molecular hydrogen and hydroxide ion. Solvated electrons are also involved in electrode processes. The diffusivity of the solvated electron in liquid ammonia can be determined using potential-step chronoamperometry. Oct 22, 2020 The sodium atom has a total of 11 electrons, so we have to put 11 electrons in orbitals. The first two electrons will go in the 1s orbital as S orbital can hold a maximum of two electrons only. The next two will go in 2s orbital and the next six electrons will go in 2p orbital as P orbital can only hold a maximum of 6 electrons.
A solvated electron is a freeelectron in (solvated in) a solution, and is the smallest possible anion. Solvated electrons occur widely, although it is difficult to observe them directly because their lifetimes are so short.[1] The deep color of solutions of alkali metals in liquid ammonia arises from the presence of solvated electrons: blue when dilute and copper-colored when more concentrated (> 3 molar).[2] Classically, discussions of solvated electrons focus on their solutions in ammonia, which are stable for days, but solvated electrons also occur in water and other solvents – in fact, in any solvent that mediates outer-sphere electron transfer. The real hydration energy of the solvated electron can be estimated by using the hydration energy of a proton in water combined with kinetic data from pulse radiolysis experiments. The solvated electron forms an acid–base pair with atomic hydrogen.
The solvated electron is responsible for a great deal of radiation chemistry.
Alkali metals dissolve in liquid ammonia giving deep blue solutions, which conduct electricity. The blue colour of the solution is due to ammoniated electrons, which absorb energy in the visible region of light. Alkali metals also dissolve in some small primary amines, such as methylamine and ethylamine[3] and hexamethylphosphoramide, forming blue solutions. Solvated electron solutions of the alkaline earth metals magnesium, calcium, strontium and barium in ethylenediamine have been used to intercalate graphite with these metals.[4]
History[edit]
The observation of the color of metal-electride solutions is generally attributed to Humphry Davy. In 1807–1809, he examined the addition of grains of potassium to gaseous ammonia (liquefaction of ammonia was invented in 1823). James Ballantyne Hannay and J. Hogarth repeated the experiments with sodium in 1879–1880. W. Weyl in 1844 and C. A. Seely in 1871 used liquid ammonia while Hamilton Cady in 1897 related the ionizing properties of ammonia to that of water. Charles A. Kraus measured the electrical conductance of metal ammonia solutions and in 1907 attributed it to the electrons liberated from the metal.[5][6] In 1918, G. E. Gibson and W. L. Argo introduced the solvated electron concept.[7] They noted based on absorption spectra that different metals and different solvents (methylamine, ethylamine) produce the same blue color, attributed to a common species, the solvated electron. In the 1970s, solid salts containing electrons as the anion were characterized.[8]
Properties[edit]
Focusing on solutions in ammonia, liquid ammonia will dissolve all of the alkali metals and other electropositive metals such as Ca,[9]Sr, Ba, Eu, and Yb (also Mg using an electrolytic process[10]), giving characteristic blue solutions.

A lithium–ammonia solution at −60 °C is saturated at about 15 mol% metal (MPM). When the concentration is increased in this range electrical conductivity increases from 10−2 to 104ohm−1cm−1 (larger than liquid mercury). At around 8 MPM, a 'transition to the metallic state' (TMS) takes place (also called a 'metal-to-nonmetal transition' (MNMT)). At 4 MPM a liquid-liquid phase separation takes place: the less dense gold-colored phase becomes immiscible from a denser blue phase. Above 8 MPM the solution is bronze/gold-colored. In the same concentration range the overall density decreases by 30%.
Sodium Electrons Gained
Dilute solutions are paramagnetic and at around 0.5 MPM all electrons are paired up and the solution becomes diamagnetic. Several models exist to describe the spin-paired species: as an ion trimer; as an ion-triple—a cluster of two single-electron solvated-electron species in association with a cation; or as a cluster of two solvated electrons and two solvated cations.
Solvated electrons produced by dissolution of reducing metals in ammonia and amines are the anions of salts called electrides. Such salts can be isolated by the addition of macrocyclicligands such as crown ether and cryptands. These ligands bind strongly the cations and prevent their re-reduction by the electron.
In neutral of partially-oxidized metal-ammonia or metal-aqua complexes diffuse solvated electrons are present. These species are recognized as 'Solvated electron precursors' (SEPs). Simply a SEP is a metal complex that bear diffuse electrons in the periphery of the ligands.[11] The diffuse solvated electron cloud occupies a quasi-spherical atomic s-type orbital and populate higher angular momentum p-, d-, f-, g-type orbitals in excited states.[12][13][14][15]
Its standard electrode potential value is -2.77 V.[16] Equivalent conductivity 177 Mho cm2 is similar to that of hydroxide ion. This value of equivalent conductivity corresponds to a diffusivity of 4,75*10−5 cm2s−1.[17]
Some thermodynamic properties of the solvated electron have been investigated by Joshua Jortner and Richard M. Noyes (1966)[18]
Alkaline aqueous solutions above pH = 9.6 regenerate the hydrated electron through the reaction of hydrated atomic hydrogen with hydroxide ion giving water beside hydrated electrons.
Below pH = 9.6 the hydrated electron reacts with the hydronium ion giving atomic hydrogen, which in turn can react with the hydrated electron giving hydroxide ion and usual molecular hydrogen H2.
The properties of solvated electron can be investigated using the rotating ring-disk electrode.
Reactivity and applications[edit]
The solvated electron reacts with oxygen to form a superoxideradical (O2.−).[19] With nitrous oxide, solvated electrons react to form hydroxyl radicals (HO.).[20] The solvated electrons can be scavenged from both aqueous and organic systems with nitrobenzene or sulfur hexafluoride[citation needed].
A common use of sodium dissolved in liquid ammonia is the Birch reduction. Other reactions where sodium is used as a reducing agent also are assumed to involve solvated electrons, e.g. the use of sodium in ethanol as in the Bouveault–Blanc reduction.
Solvated electrons are involved in the reaction of sodium metal with water.[21] Two solvated electrons combine to form molecular hydrogen and hydroxide ion.
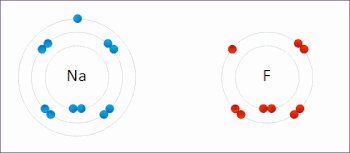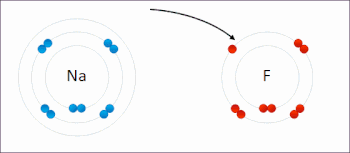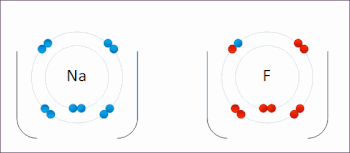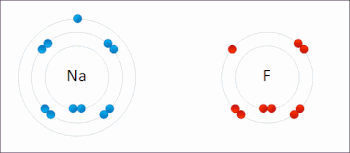
Sodium Electrons Bohr Model
Solvated electrons are also involved in electrode processes.[22]
Sodium Electrons Gain
Diffusion[edit]
The diffusivity of the solvated electron in liquid ammonia can be determined using potential-step chronoamperometry.[23]
In gas phase and upper atmosphere of Earth[edit]
Solvated electrons can be found even in the gas phase. This implies their possible existence in the upper atmosphere of Earth and involvement in nucleation and aerosol formation.[24]
References[edit]
- ^Schindewolf, U. (1968). 'Formation and Properties of Solvated Electrons'. Angewandte Chemie International Edition in English. 7 (3): 190–203. doi:10.1002/anie.196801901.
- ^Cotton, F. A.; Wilkinson, G. (1972). Advanced Inorganic Chemistry. John Wiley and Sons Inc. ISBN978-0-471-17560-5.
- ^Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^W. Xu and M. M. Lerner, 'A New and Facile Route Using Electride Solutions To Intercalate Alkaline Earth Ions into Graphite', Chem. Mater. 2018, 30, 19, 6930–6935. https://doi.org/10.1021/acs.chemmater.8b03421
- ^Kraus, Charles A. (1907). 'Solutions of Metals in Non-Metallic Solvents; I. General Properties of Solutions of Metals in Liquid Ammonia'. J. Am. Chem. Soc.29 (11): 1557–1571. doi:10.1021/ja01965a003.
- ^Zurek, Eva (2009). 'A Molecular Perspective on Lithium–Ammonia Solutions'. Angew. Chem. Int. Ed.48 (44): 8198–8232. doi:10.1002/anie.200900373. PMID19821473.
- ^Gibson, G. E.; Argo, W. L. (1918). 'The Absorption Spectra of the Blue Solutions of Certain Alkali and Alkaline Earth Metals in Liquid Ammonia and Methylamine'. J. Am. Chem. Soc.40 (9): 1327–1361. doi:10.1021/ja02242a003.
- ^Dye, J. L. (2003). 'Electrons as Anions'. Science. 301 (5633): 607–608. doi:10.1126/science.1088103. PMID12893933.
- ^Edwin M. Kaiser (2001). 'Calcium-Ammonia'. Calcium–Ammonia. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc003. ISBN978-0471936237.
- ^Combellas, C; Kanoufi, F; Thiébault, A (2001). 'Solutions of solvated electrons in liquid ammonia'. Journal of Electroanalytical Chemistry. 499: 144–151. doi:10.1016/S0022-0728(00)00504-0.
- ^Ariyarathna, Isuru R.; Pawłowski, Filip; Ortiz, Joseph Vincent; Miliordos, Evangelos (2018). 'Molecules mimicking atoms: monomers and dimers of alkali metal solvated electron precursors'. Physical Chemistry Chemical Physics. 20 (37): 24186–24191. doi:10.1039/c8cp05497e.
- ^Ariyarathna, Isuru R.; Khan, Shahriar N.; Pawłowski, Filip; Ortiz, Joseph Vincent; Miliordos, Evangelos (4 January 2018). 'Aufbau Rules for Solvated Electron Precursors: Be(NH 3 ) 4 0,± Complexes and Beyond'. The Journal of Physical Chemistry Letters. 9 (1): 84–88. doi:10.1021/acs.jpclett.7b03000.
- ^Ariyarathna, Isuru R.; Miliordos, Evangelos (2019). 'Superatomic nature of alkaline earth metal–water complexes: the cases of Be(H 2 O)0,+4 and Mg(H 2 O)0,+6'. Physical Chemistry Chemical Physics. 21 (28): 15861–15870. doi:10.1039/c9cp01897b.
- ^Ariyarathna, Isuru R.; Miliordos, Evangelos (2020). 'Geometric and electronic structure analysis of calcium water complexes with one and two solvation shells'. Physical Chemistry Chemical Physics. 22 (39): 22426–22435. doi:10.1039/d0cp04309e.
- ^Ariyarathna, Isuru (1 March 2021). 'First Principle Studies on Ground and Excited Electronic States: Chemical Bonding in Main-Group Molecules, Molecular Systems with Diffuse Electrons, and Water Activation using Transition Metal Monoxides'.
- ^Baxendale, J. H. (1964), Radiation Res. Suppl., 114 and 139
- ^Hart, Edwin J. (1969). 'The Hydrated Electron'. Survey of Progress in Chemistry. 5: 129–184. doi:10.1016/B978-0-12-395706-1.50010-8. ISBN9780123957061.
- ^Jortner, Joshua; Noyes, Richard M. (1966). 'Some Thermodynamic Properties of the Hydrated Electron'. The Journal of Physical Chemistry. 70 (3): 770–774. doi:10.1021/j100875a026.
- ^Hayyan, Maan; Hashim, Mohd Ali; Alnashef, Inas M. (2016). 'Superoxide Ion: Generation and Chemical Implications'. Chemical Reviews. 116 (5): 3029–3085. doi:10.1021/acs.chemrev.5b00407. PMID26875845.
- ^Janata, Eberhard; Schuler, Robert H. (1982). 'Rate constant for scavenging eaq- in nitrous oxide-saturated solutions'. The Journal of Physical Chemistry. 86 (11): 2078–2084. doi:10.1021/j100208a035.
- ^Walker, D.C. (1966). 'Production of hydrated electron'. Canadian Journal of Chemistry. 44 (18): 2226–. doi:10.1139/v66-336.
- ^B. E. Conway, D. J. MacKinnon, J. Phys. Chem., 74, 3663, 1970
- ^Harima, Yutaka; Aoyagui, Shigeru (1980). 'The diffusion coefficient of solvated electrons in liquid ammonia'. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 109 (1–3): 167–177. doi:10.1016/S0022-0728(80)80115-X.
- ^F. Arnold, Nature 294, 732-733, (1981)
Further reading[edit]
- Sagar, D. M.; Colin; Bain, D.; Verlet, Jan R. R. (2010). 'Hydrated Electrons at the Water/Air Interface'. J. Am. Chem. Soc. 132 (20): 6917–6919. doi:10.1021/ja101176r. PMID20433171.
- Martyna, Glenn (1993). 'Electronic states in metal-ammonia solutions'. Physical Review Letters. 71 (2): 267–270. Bibcode:1993PhRvL..71..267D. doi:10.1103/physrevlett.71.267. PMID10054906.
- Martyna, Glenn (1993). 'Quantum simulation studies of singlet and triplet bipolarons in liquid ammonia'. Journal of Chemical Physics. 98 (1): 555–563. Bibcode:1993JChPh..98..555M. doi:10.1063/1.464650.
- Solvated Electron. Advances in Chemistry. 50. 1965. doi:10.1021/ba-1965-0050. ISBN978-0-8412-0051-7.
- Anbar, Michael (1965). 'Reactions of the Hydrated Electron'. Solvated Electron. Advances in Chemistry. 50. pp. 55–81. doi:10.1021/ba-1965-0050.ch006. ISBN978-0-8412-0051-7.
- Abel, B.; Buck, U.; Sobolewski, A. L.; Domcke, W. (2012). 'On the nature and signatures of the solvated electron in water'. Phys. Chem. Chem. Phys. 14 (1): 22–34. Bibcode:2012PCCP...14...22A. doi:10.1039/C1CP21803D. PMID22075842.
- Harima, Y.; Aoyagui, S. (1981). 'Determination of the chemical solvation energy of the solvated electron'. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 129 (1–2): 349–352. doi:10.1016/S0022-0728(81)80027-7.
- Hart, Edwin J. (1969). 'The Hydrated Electron'. Survey of Progress in Chemistry Volume 5. Survey of Progress in Chemistry. 5. pp. 129–184. doi:10.1016/B978-0-12-395706-1.50010-8. ISBN9780123957061.
- The electrochemistry of the solvated electron. Technische Universiteit Eindhoven.
- IAEA On the Electrolytic Generation of Hydrated Electron.
- Fundamentals of Radiation Chemistry, chapter 6, p. 145–198, Academic Press, 1999.
- Tables of bimolecular rate constants of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds International Journal of Applied Radiation and Isotopes Anbar, Neta


